Home
Dimitri Mendeleev and the History of the Periodic Table
Read about the History of the Periodic Table. Dimitri Mendeleev was born on February 7th 1834 in Tobolsk, a Town in Siberia. In 1869 at the age of 35 the famous Russian Scientist perceived a totally new classification Method “the periodic table”, he included all the 65 elements known in his time by their atomic weights and chemical valency. Mendeleev then went even further, using the remaining gaps and spaces in his periodic table, he correctly concluded that a further group of yet unknown elements must exist in order to fill in the gaps in his Periodic Table, this group we now know as the lanthanides, and is Group six of our modern Standardised Periodic Table.
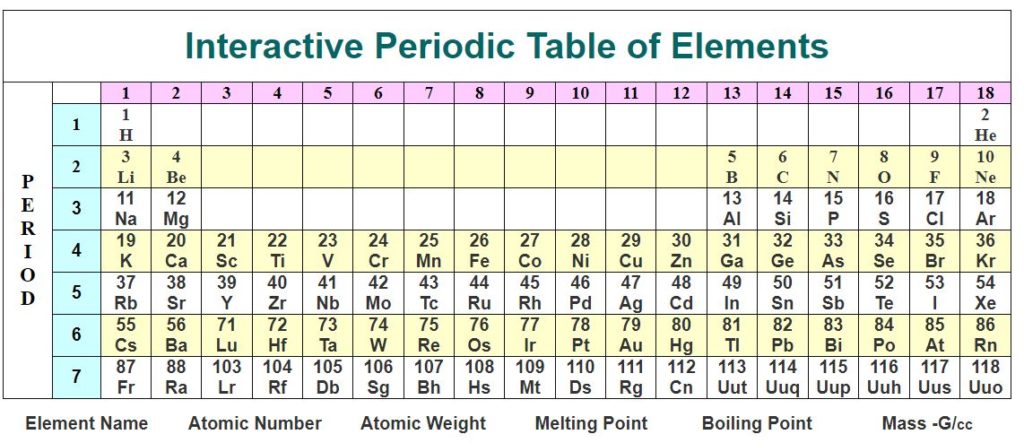
The Periodic Table – Interactive Periodic Table
Fifty years after Dimitri Mendeleev created the Periodic cable, the British scientist Henry Moseley discovered that the number of protons in the nucleus of a particular type of atom was always the same. When atoms are arranged via their Atomic Number, the few remaining problems with Mendeleev’s original periodic table disappeared. Due to Moseley’s work, the modern periodic table is based on the atomic numbers of the elements rather than atomic mass.
Dimitri Mendeleev’s work on the Periodic Table chart recognised
Dimitri Mendeleev has clearly left his mark on modern science, indeed all modern Scientists are familiar with Standardised version of his Periodic table. Mendeleyev’s homeland, Russia, has recognised the significance of his work by naming the “Mendeleyev University of Chemical Technology” in Moscow in his honour.
The Periodic Table IUPAC and the modern standardised Periodic Table Chart
The standardised periodic table in use today was agreed by the International Union of Pure Applied Chemistry, IUPAC, in 1985 and now recognises more periods and elements than Dimitri Mendeleev knew in his day in his day but still all fitting into his concept of the “Periodic Table”.
The Interactive Periodic Table – Revision and Homework Help
Test your knowledge of chemistry and the Periodic Table by completing the Element Symbols and Atomic Numbers for all of the elements. Click the following link to Blank Periodic Table and print! Start practising – the more you fill in the empty spaces the more you will remember. Repetition is the key to good knowledge retention.